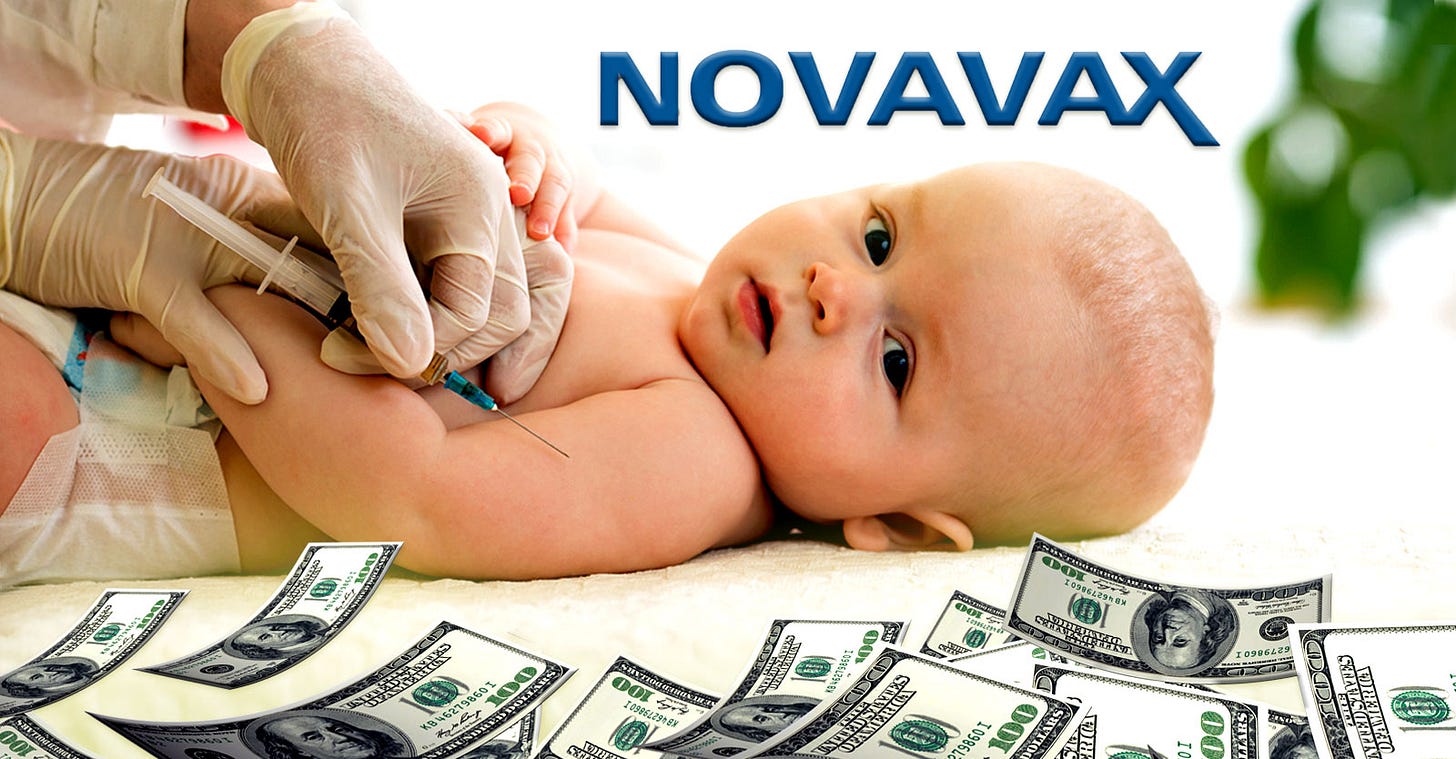
$3,000 and a Toy: Novavax Dangles Incentives to Fill Clinical Trials on COVID Shots for Babies, Kids
The “Hummingbird” trial is testing two primary shots and a booster shot of Novavax’s adjuvanted recombinant spike protein nanoparticle vaccine. Novavax is offering parents up to $3,000 to enroll.
Novavax is offering parents up to $3,000 to enroll their children in the vaccine maker’s Phase 2/3 COVID-19 vaccine trial for infants and children ages 6 months to 11 years. The offer also includes a stuffed animal for each child.
The “Hummingbird” trial is testing two primary shots and a booster shot of Novavax’s adjuvanted recombinant spike protein nanoparticle vaccine in children. The study, which began in 2022, is enrolling 3,600 children.
The study, which began in the U.S., is expected to run through 2025 and will be conducted in the U.S. and other countries.
The children will receive three injections and visit the clinic eight times. Parents will participate in three phone calls and keep an e-diary of the vaccine’s effects on their child. Some children will receive two additional injections, for a total of five shots.
The study website promises, “You will be compensated for your time and travel regardless of your immigration status. Transportation to the study site may also be provided, as available. No health insurance is required to participate.”
Recruitment materials from Be Well Clinical Studies, which is running one of the U.S. trials, state that compensation can be more than $3,000 over two years.
A 2023 video explaining the study also promises incentives for the children, including “a Covid stuffed animal.”
In the video, a pastor from Louisiana who has four children enrolled in the study said incentives like the stuffed animal made the kids even “more excited than the parents” to participate.
The video features Dr. Jibran Atwi who is running a Hummingbird trial in Lafayette, Louisiana. He encouraged people to participate in the study, because COVID-19 severely affected kids, particularly through lockdowns and lost schooling.
Atwi also said that COVID-19 can be “very disruptive” because if a child has to stay home from school, parents may not be able to go work and the child may have to be isolated from their grandparents.
“Prevention,” he said, “is the best medicine.” He added that there had been an “impressive response” from parents who wanted to participate.
Research shows that young children rarely get sick from COVID-19 and that the illness is typically mild in older kids.
Atwi received over $2 million in research funding in 2023 from Big Pharma, according to the Open Payments website.
Most of that funding came from Genzyme — a Sanofi subsidiary — and from Sanofi, which shares the co-exclusive licensing agreement with Novavax to commercialize its COVID-19 vaccine.
In 2022, Atwi received over $1 million, largely from AstraZeneca and Genzyme.
Last week, the U.S. Food and Drug Administration (FDA) granted emergency use authorization to Pfizer and Moderna’s 2024-2025 mRNA COVID-19 shots, but Novavax’s 2024-2025 formula has not yet been authorized.
The FDA has authorized previous versions of the Novavax vaccine, but only for children ages 12 and up.
High payments place children ‘at risk of coercion’
Other pharmaceutical companies that have paid research subjects large sums of money have come under scrutiny. In the United Kingdom (U.K.), Moderna was criticized for initially offering children’s families 1,505 pounds ($1,984 dollars) to participate in its NextCOVE clinical trial, which is testing Moderna’s mRNA vaccine in children ages 12 and up.
The Children’s Covid Vaccine Advisory Council submitted a complaint to the U.K.’s Prescription Medicines Code of Practice Authority (PMCPA) — an industry trade group that regulates ethical practices — raising concerns about “inappropriate financial inducement” offered to children and their parents to participate in the trial.
The council cited concerns raised by the research ethics committee (REC) that approved the clinical study. Regarding the 1,500-pound payment they wrote:
“This amount seems much higher than what would be considered a reasonable reimbursement and therefore would contravene clinical trial regulations. The Medicines for Human Use (Clinical Trials) Regulations (2004) explicitly prohibit the giving of incentives or financial inducements to children…..or their parents.”
The REC said the amount, “placed the children at risk of coercion,” and the organization required that Moderna reduce the offer before recruitment could begin. Moderna reduced the amount to 185 pounds ($244 dollars).
Yet, according to the complaint, at least one pediatrician continued to offer the high enrollment compensation.
The PMCPA sanctioned Moderna, and the case report on the issue is currently pending.
If the PMCPA determines a pharmaceutical company has breached the industry code, it can require the company to pay administrative charges or issue a corrective statement. Or, it may request a compulsory audit of the company.
In the U.S., Be Well is also advertising that it will pay parents $2,400 for enrolling their infants and toddlers, ages 5-23 months, in Moderna’s Rhyme Trial for an mRNA RSV and a human metapneumovirus (hMPV) vaccine.
According to the clinicaltrials.gov website, Be Well withdrew from the Moderna RSV study, but the website is still advertising to recruit participants.
Be Well is run by founder and director Dr. Mark Carlson, a geriatrician, who has taken nearly $3 million in research funding from Big Pharma, mostly from Moderna, since 2021.
Moderna did not respond to The Defender’s inquiry about compensation offered to children’s families to participate in these studies.






I guess you could put the stuffed toy in the coffin.
Vaccine industry establishment figures routinely repeat the line that we "can't" do the placebo-controlled clinical trials which were not done for most of the "established" vaccine products because "we" can't "ethically" deny administering the products in question for the duration of such a study since they "workTM". They appear to have no concern whatsoever about the ethics of exploiting financial precariousness and administering experimental products to children.